Breast cancer is a battle that affects one in eight women globally. For many, a mastectomy - a surgical procedure that removes the affected breast tissue - is a necessary part of treatment.
Each year, an estimated 600,000 mastectomies are performed, yet only around 30 per cent of those women undergo breast reconstruction surgery - a disparity that’s thought to be down to a mix of factors including a reluctance to undergo further invasive surgical procedures and concerns over longer-term complications.
French tissue engineering specialist Lattice Medical hopes to change all that with a reconstructive process based on its 3D printed Matisse device, a solution that Lattice R&D Manager Jaime Destousse claims offers a less invasive, more natural, and longer-lasting alternative to traditional methods of breast reconstruction.
We want this device to become the gold standard in breast reconstruction
Jaime Destousse - R&D Manager, Lattice Medical
Currently, two primary methods dominate breast reconstruction surgery: silicone breast implants and autologous reconstruction. Silicone implants, while the most common, offer a quick solution but come with significant drawbacks, not least the fact that having a long-term foreign body implanted can induce medical complications. These implants also typically need to be replaced every 10 to 15 years, which can be off-putting for some patients.
Autologous reconstruction involves either Lipofilling (harvesting fat from another part of the a patient’s body to recreate the breast) or flap reconstruction, a reconstructive technique where a flap of tissue from the patient’s own body – usually taken from the abdomen or back – is transferred to the breast area where it’s blood vessels are connected to blood vessels in chest.
Destousse said that whilst these approaches lead to a more natural looking results they are both long and arduous processes, requiring multiple interventions and often leading to back pain and loss of strength at the donor site, making it an unattractive option for many women.
The Matisse device, he claims, combines the advantages of these techniques, but removes many of the obstacles that lead to patients opting out of reconstructive surgery. “Our device is minimally invasive, natural, and long-lasting,” he said.
Underpinned by six years of research at Lille University hospital, the device is made up of two components: a scaffold and a hemispherical chamber. Both components are made from resorbable, biocompatible materials, meaning that after serving their purpose, they safely degrade and disappear within 18 to 24 months, leaving the body without the need for additional surgeries.
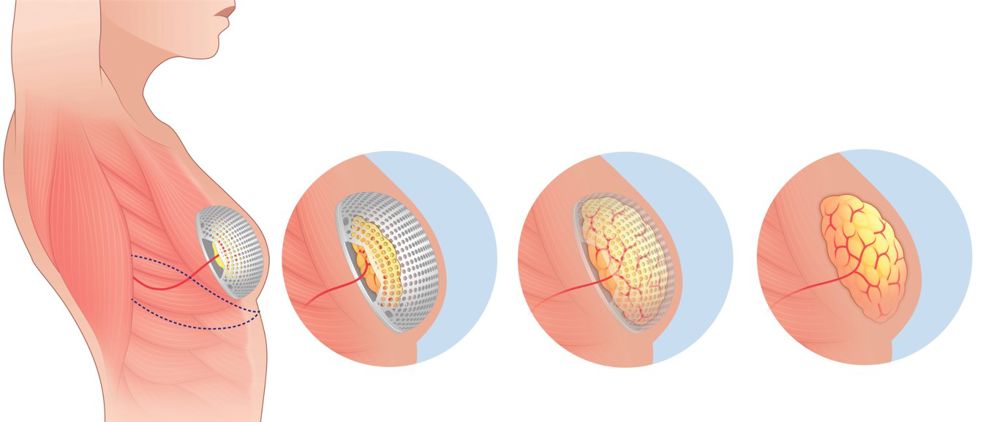
Using cutting-edge 3D printing techniques, Lattice has developed a core range of Matisse implants in 21 sizes, while also offering custom-made options to suit individual patients. Initially, the team experimented with direct drive extrusion from pellets to print the devices, but soon realised the extended exposure to heat affected the physical and chemical properties of the materials. After refining their approach, they settled on using FDM (fused deposition modelling) technology, transforming pellets into filaments before printing. “With some modifications, we were able to adapt the technique for medical devices, and it worked really well,” Destousse said.
One of the standout features of Lattice Medical’s operation is its attention to the sterility and precision of the production process. The Matisse devices are manufactured in a state-of-the-art clean room facility in France, where strict protocols are followed to control temperature, particle contamination, and sterilisation. “We are one of the first 3D printing firms in Europe to produce medical devices in a clean room,” noted Destousse.
With 16 3D printers currently operational, the company is capable of producing 1,000 implants per year, and plans are in place to double that capacity.
The innovation behind the Matisse device extends beyond the implant itself. The surgical procedure is designed to be as minimally invasive as possible, offering a significant improvement over existing techniques. In the procedure, a surgeon harvests a small pedicle of fat from near the breast area, which is then placed inside the Matisse device and sutured to the base. Once assembled, the device is implanted in the patient, and the tissue begins to grow inside the chamber. “Using this technique, reconstruction takes between six and nine months, and the material degrades harmlessly within 18 to 24 months,” said Destousse.
The device operates through a physiological mechanism that allows the fat to grow without any mechanical stress. The body’s natural inflammatory response enhances the growth of fat tissue, promoting a more effective and quicker recovery. “It’s like when you do exercise. if you don’t stress the fat, it will grow,” explained Destousse.
Before moving into human trials, Lattice Medical conducted successful preclinical studies on animal models, including nine mini-pigs, a breed of small pig commonly used for medical testing.
The results were promising: within three to six months, the volume of the device was fully filled with fat tissue, and after 21 months, that volume remained stable. “The biological mechanisms in these animals are very similar to those in humans, which gives us a high level of confidence that the procedure will be both safe and effective,” said Destousse.

The device is now undergoing human clinical trials which will involve 50 patients across France, Spain, and Georgia over the course of 36 months. Initial results have been encouraging, with the small number of early patients reporting low levels of pain and a smooth recovery process. “We follow up with the patients every six months to monitor their health and the performance of the device,” says Destousse.
Looking ahead, the company is aiming to secure CE marking in Europe by 2026, which would allow it to commercialise Matisse across the continent. Expansion into the UK, particularly through partnerships with the NHS, is also on the horizon. Furthermore, Lattice Medical is laying the groundwork for clinical trials in the United States, with hopes of eventually bringing its product to the American market.
In addition to breast reconstruction, Lattice Medical is developing a skin reconstruction product, which is currently in preclinical stages.
In the meantime, by combining the best aspects of existing breast reconstruction techniques while eliminating their downsides, the company looks set to make breast reconstruction more accessible, less traumatic, and ultimately more effective for a larger number of women. “We want this device to become the gold standard in breast reconstruction,” Destousse said. And with the promising results achieved so far, that goal may soon be within reach.












McMurtry Spéirling defies gravity using fan downforce
Ground effect fans were banned from competitive motorsport from the end of the 1978 season following the introduction of Gordon Murray's Brabham...