Led by Huanyu “Larry” Cheng, Dorothy Quiggle Career Development Professor in Penn State University's Department of Engineering Science and Mechanics, the researchers have published details of the device in Biosensors and Bioelectronics.
The researchers constructed the device first with laser-induced graphene (LIG), a material consisting of atom-thick carbon layers in various shapes.
Insulin-delivery patch monitors and manages glucose levels
Wearable sensor measures glucose without need for finger-prick blood test
“The challenge here is that LIG is not sensitive to glucose at all,” Cheng said in a statement. “So, we needed to deposit a glucose-sensitive material onto the LIG.”
The team chose nickel because of its robust glucose sensitivity, according to Cheng, and combined it with gold to lower potential risks of an allergic reaction. The researchers hypothesised that the LIG outfitted with the nickel-gold alloy would be able to detect low concentrations of glucose in sweat on the skin’s surface.
According to Cheng, there is a strong correlation between glucose levels in sweat and blood. While the concentration of glucose in sweat is about 100 times less than the concentration in blood, the team’s device is sensitive enough to accurately measure the glucose in sweat and reflect the concentration in blood.
The nickel-gold alloy’s sensitivity allowed Cheng’s team to exclude enzymes, which are often used to measure glucose in more invasive, commercially available devices or in non-invasive monitors proposed by other researchers. These enzymes, however, can degrade quickly with time and changing temperatures.
“An enzymatic sensor has to be kept at a certain temperature and pH, and the enzyme can’t be stored in the long term,” Cheng said. “A non-enzymatic glucose sensor, on the other hand, is advantageous in terms of stable performance and glucose sensitivity regardless of these changes.”
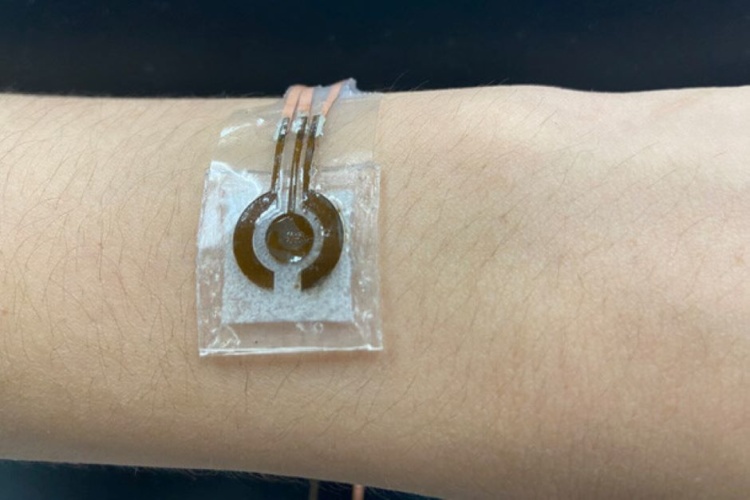
Non-enzymatic sensors require alkaline solution, which can damage the skin and typically limits device wearability. To curb this issue, Cheng and his team attached a microfluidic chamber to the LIG alloy. This chamber is smaller than previously developed configurations to promote wearability and porous to allow for a range of movement. It is connected to a collection inlet that passes sweat into the solution without allowing the solution to touch the skin. The basic solution interacts with the glucose molecules to produce a compound that reacts with the alloy. This reaction triggers an electrical signal, indicating the concentration of glucose in the sweat.
With a smaller alkaline solution chamber, the entire device is roughly the size of a quarter coin and is flexible enough to maintain a secure attachment to the human body, Cheng said.
In a proof-of-concept test, the researchers used a skin-safe adhesive to attach the reusable device to a person’s arm one hour and three hours after a meal. The subject performed a brief workout to produce sweat before each measurement. A few minutes after collecting the sweat, the researchers found that the detected glucose concentration dropped from the first measurement to the next. The glucose measurements from the device were verified by measurements made with a commercially available glucose monitor.
Cheng and the team plan to improve upon their prototype for future applications, including addressing how patients or clinicians may use the sensor for incremental glucose measurements or continuous monitoring to determine treatment. They also intend to refine and expand this platform for more comfortable monitoring of other biomarkers that can be found in the sweat or interstitial fluids.










The economy cannot wait for next gen AI and digital talent
If I recall my Greek mythology correctly, Cassandra received the gift of prophecy but was cursed to never be believed … don´t know how much that...