Novel carbon storage technique ‘fastest of its kind’
Researchers from the University of Texas at Austin have developed new carbon storage technology that works faster than current methods and without the harmful chemical accelerants.
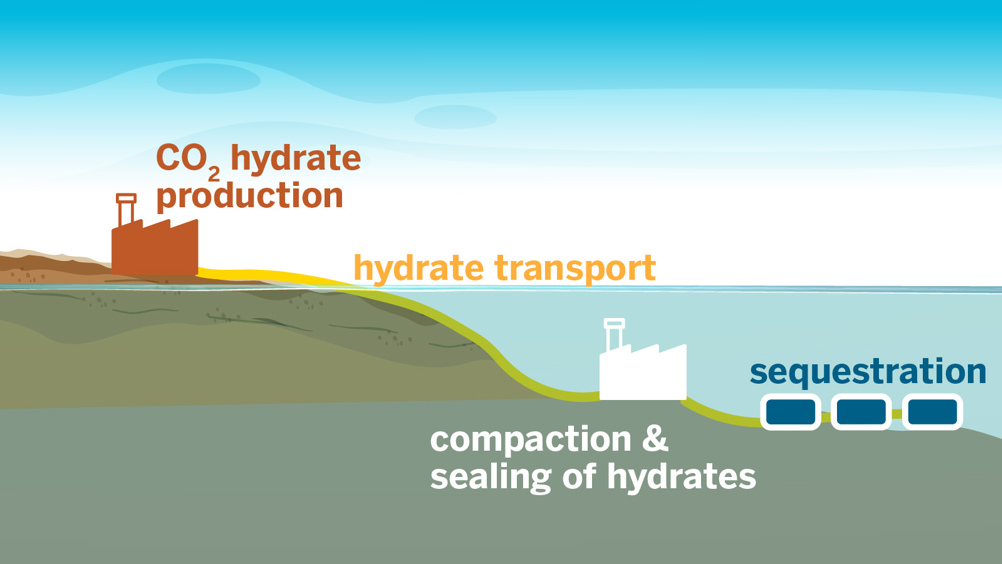
In the new study, published in ACS Sustainable Chemistry & Engineering, the researchers developed a technique for ultrafast formation of carbon dioxide hydrates.
According to the researchers, the unique ice-like materials can bury carbon dioxide in the ocean, preventing it from being released into the atmosphere.
“We’re staring at a huge challenge – finding a way to safely remove gigatons of carbon from our atmosphere – and hydrates offer a universal solution for carbon storage. We need the technology to grow them rapidly and at scale,” Vaibhav Bahadur, a professor in the Walker Department of Mechanical Engineering and research lead said in a statement. “We’ve shown that we can quickly grow hydrates without using any chemicals that offset the environmental benefits of carbon capture.”
Currently, the most common carbon storage method involves injecting CO2 into underground reservoirs. This technique has the dual benefits of trapping carbon and increasing oil production.
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










Water Sector Talent Exodus Could Cripple The Sector
Maybe if things are essential for the running of a country and we want to pay a fair price we should be running these utilities on a not for profit...