New reactor could decarbonise ammonia production
Ammonia production could be decarbonised – and water pollution mitigated – with a reactor design developed by engineers at Rice University, Texas.
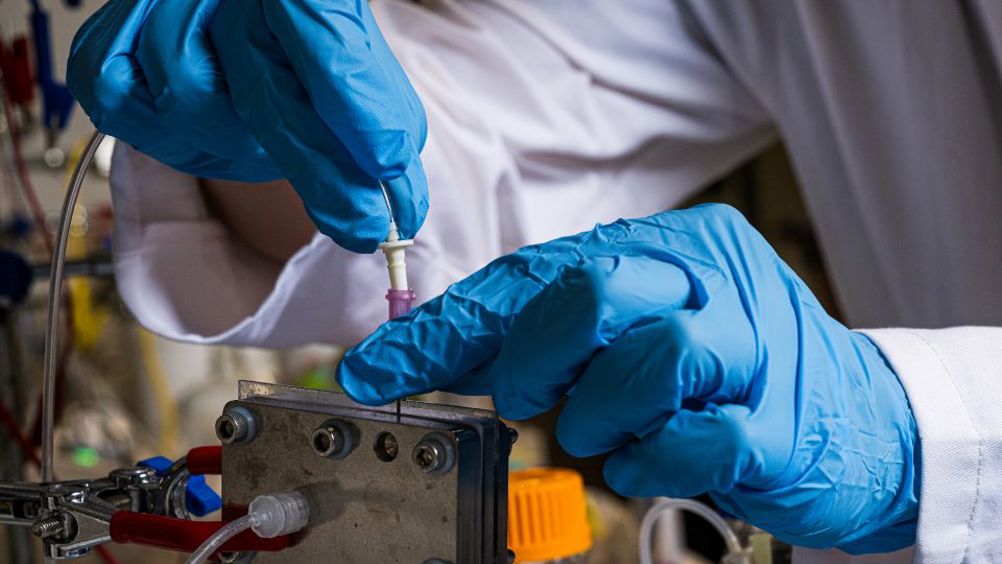
In a study published in Nature Catalysis, Rice engineers led by Haotian Wang described the development of a new reactor system that converts nitrates into ammonia, a chemical used in fertilisers and a range of industrial and commercial products.
Ammonia is one of the most widely produced chemicals globally, with worldwide demand surpassing 180 million tons annually.
The main way to make ammonia is the Haber-Bosch process, a chemical reaction that synthesises ammonia (NH3) from nitrogen (N2) and hydrogen (H2) gases that occurs under high temperature and pressure conditions and is dependent on large-scale centralised infrastructure.
One alternative to this process is electrochemical synthesis, which uses electricity to drive chemical reactions.
In a statement, Feng-Yang Chen, a Rice graduate student and lead author, said: “Electrochemistry can occur at room temperature, is more amenable to scalable formats for different infrastructure systems, and has the capacity to be powered by decentralised renewable energy. However, the current challenge for this technology is that large quantities of additive chemicals are required during the electrochemical conversion process. The reactor we developed uses recyclable ions and a three-chamber system to improve the reaction’s efficiency.”
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










Water Sector Talent Exodus Could Cripple The Sector
Maybe if things are essential for the running of a country and we want to pay a fair price we should be running these utilities on a not for profit...