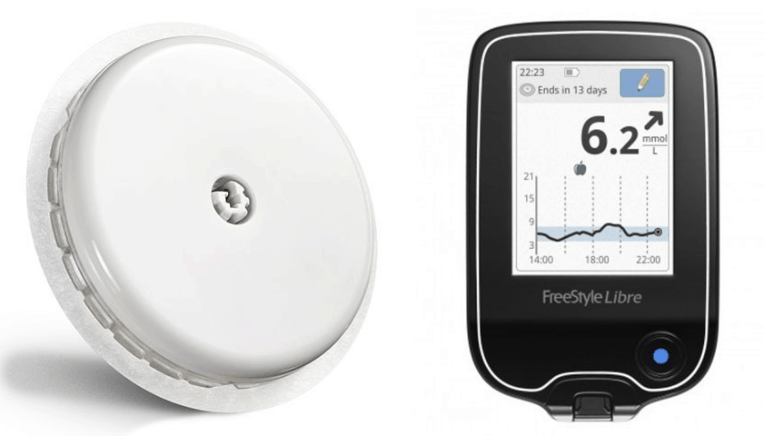
The FreeStyle Libre flash glucose monitor attaches to the upper arm and can measure blood sugar for up to 14 days. When the sensor is applied, a thin, flexible and sterile fibre is inserted just under the skin and held in place with a small adhesive pad. The small disc is designed to replace the 4-10 finger pricks Type 1 diabetes patients need to self-administer daily.
Up to eight hours of glucose data can be stored locally on the waterproof sensor, with a specialised reader used to scan the information in just one second. This data then plugs into a software system that can generate detailed reports on glucose levels, helping users and medical professionals spot patterns of spikes and lows.
“Despite major progress in the care of people living with Type 1 diabetes, many fail to achieve their target blood sugar level – and that risks major complications,” said Dr Lalantha Leelarathna, a researcher from Manchester University and a consultant diabetologist at Manchester Royal Infirmary, Manchester University NHS Foundation Trust.
“Our review concludes that the FreeStyle Libre flash glucose monitor works well for both adults and children. The studies show it is accurate, comfortable and easy to use. It is associated with a reduction in low blood sugar levels, improvements in glycated haemoglobin levels and adverse events are low.
“Our assessment of the available evidence shows that for most patients, it is a game changer.”
The monitor, which was developed by healthcare company Abbot, has been available via the NHS since November 2017. Randomised trials carried out by the research team found FreeStyle Libre use was associated with a reduction in hypoglycaemia, and also found that its accuracy was comparable to currently available real-time continuous glucose monitors. But while the benefits of the device seem clear, getting it into the hands of patients has not been straightforward.
“The challenge in the UK is to now ensure that it reaches people living with diabetes,” said Dr Emma Wilmot from Derby Teaching Hospitals.
“We are delighted that this device became available on the NHS drug tariff in November 2017. However, it is clear from the Diabetes UK map of access that this does not necessarily mean that it will make it into the hands of those who might benefit. The development of a postcode lottery for access would further add to the variation in diabetes care across the country and may adversely impact on outcomes.”










Deep Heat: The new technologies taking geothermal energy to the next level
No. Not in the UK. The one location in the UK, with the prospect of delivering heat at around 150°C and a thermal-to-electrical efficiency of 10-12%,...