The most crucial role of a cancer surgeon is to remove all traces of a tumour during the operation. Unfortunately, it's also one of the most difficult. Distinguishing between cancerous cells and healthy tissue, especially at the boundary of a tumour, is far from easy even for the most experienced surgeons, and leftover cancer cells can grow into new tumours or spread elsewhere in the body
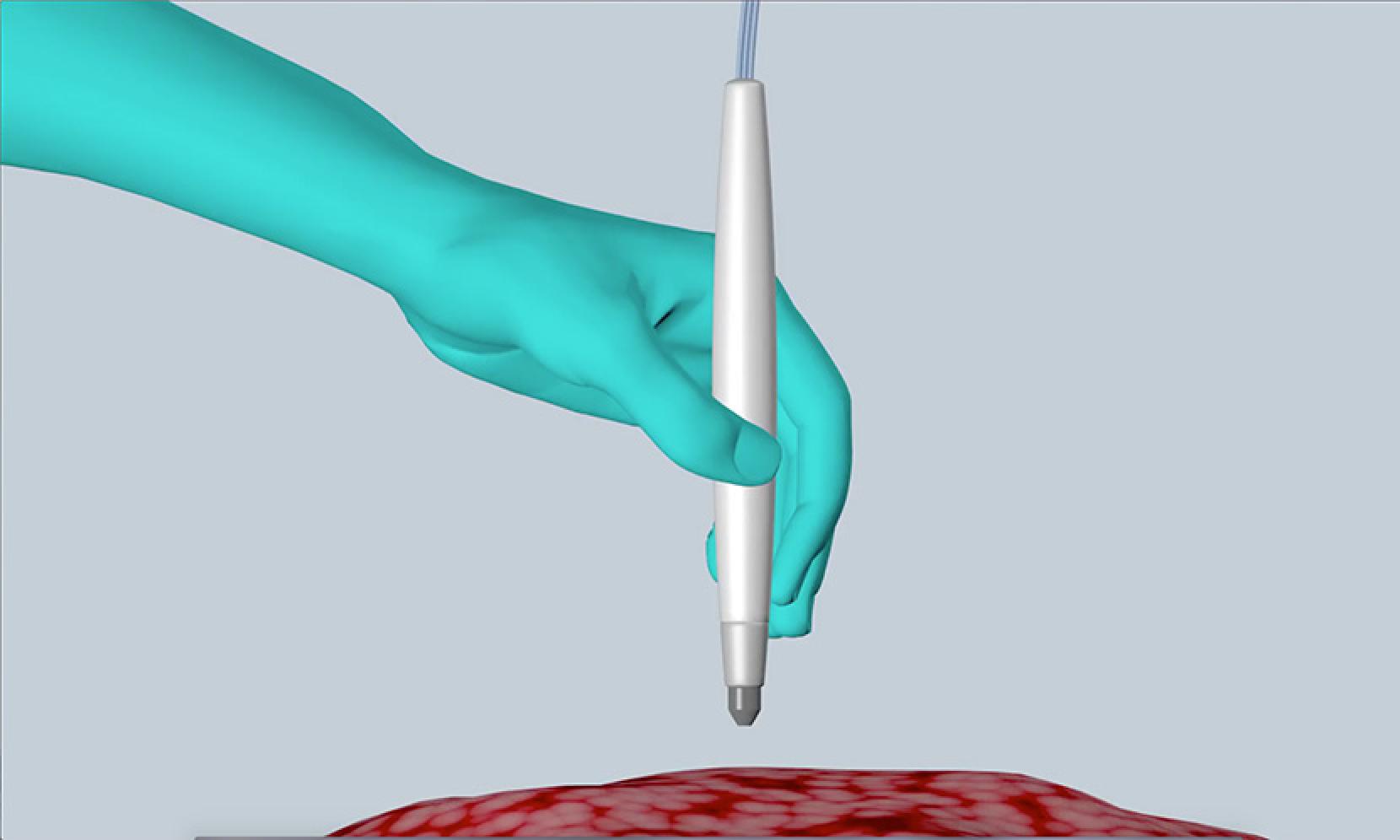
A team from the University of Texas at Austin (UT Austin) has now developed a device that can be used during surgery to give a rapid determination of whether cells at a tumour boundary are healthy or cancerous, thereby improving the accuracy and effectiveness of surgery and reducing the risk of recurrence of cancer. The size and shape of a pen, the device works on contact using the technique of mass spectrometry; the team therefore calls it a MasSpec Pen.
“If you talk to cancer patients after surgery, one of the first things many will say is ‘I hope the surgeon got all the cancer out,’ ” says Livia Schiavinato Eberlin, a chemist at UT Austin who designed the study and led the team. “It’s just heartbreaking when that’s not the case. But our technology could vastly improve the odds that surgeons really do remove every last trace of cancer during surgery.”
Currently, surgeons can use a technique called frozen section analysis during operations to determine the boundary between tumour and healthy cells. However, each sample can take 30 minutes or more to prepare and analyse, increasing the risk of infection and of problems with anaesthesia. Moreover, for some cancers this technique is unreliable.
The MasSpec pen works by analysing the molecules produced by cells during their normal processes, such as generating energy and getting rid of waste products. Known as metabolites, these molecules can be used to identify cancer cells because the cells grow out of control and their metabolisms are therefore very different from healthy cells. “Because the metabolites in cancer and normal cells are so different, we extract and analyze them with the MasSpec Pen to obtain a molecular fingerprint of the tissue,” said Eberlin. “What is incredible is that through this simple and gentle chemical process, the MasSpec Pen rapidly provides diagnostic molecular information without causing tissue damage.”
When the pen probe is placed against living tissue, it releases a drop of water that dissolves metabolites released by the tissue. The liquid is drawn back into the probe and pumped to a mass spectrometer, which analyses the dissolved molecules, compares the results to a statistical database of molecular fingerprints and sends a message to a monitor screen displaying the words "normal" or "cancer". In some types of tumour it can also determine the subtype of cancer. “When designing the MasSpec Pen, we made sure the tissue remains intact by coming into contact only with water and the plastic tip of the MasSpec Pen during the procedure,” said Jiang Zhang, who led the experimental team. “The result is a biocompatible and automated medical device that we are so excited to translate to the clinic very soon.”
In a paper in Science Translational Medicine, the team describes tests on tissue removed from 253 human cancer patients, in which the MasSpec Pen displayed greater than 96 per cent accuracy and provided results within 10 seconds, even on samples that contained a mixture of cancerous and healthy cells. The device was tested on tumours from lungs, ovaries, breast tissue and thyroid glands. The team also confirmed that the device does not harm tissue in tests on tumour-containing live mice.
The team hopes to begin clinical trials on humans in 2018. “Any time we can offer the patient a more precise surgery, a quicker surgery or a safer surgery, that’s something we want to do,” said James Suliburk, head of endocrine surgery at Baylor College of Medicine in Houston and a collaborator on the project. “This technology does all three. It allows us to be much more precise in what tissue we remove and what we leave behind.”










McMurtry Spéirling defies gravity using fan downforce
Ground effect fans were banned from competitive motorsport from the end of the 1978 season following the introduction of Gordon Murray's Brabham...